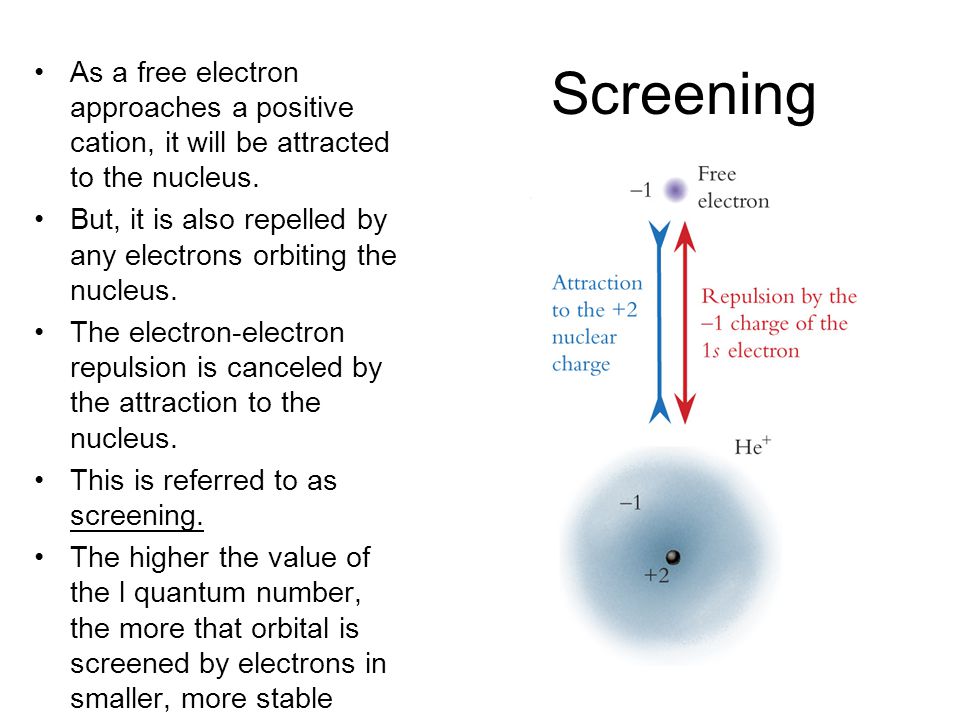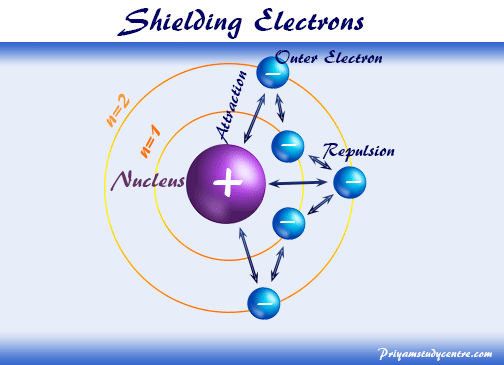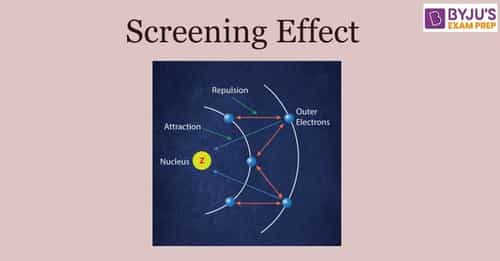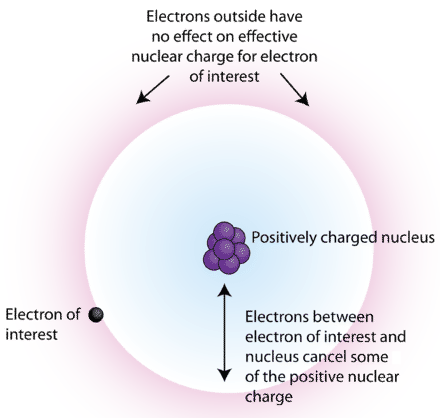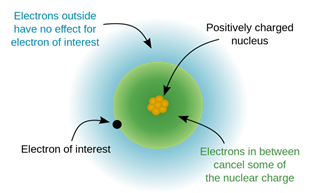
The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram

Color online) (a) Valence-electron screening cloud around the excited... | Download Scientific Diagram
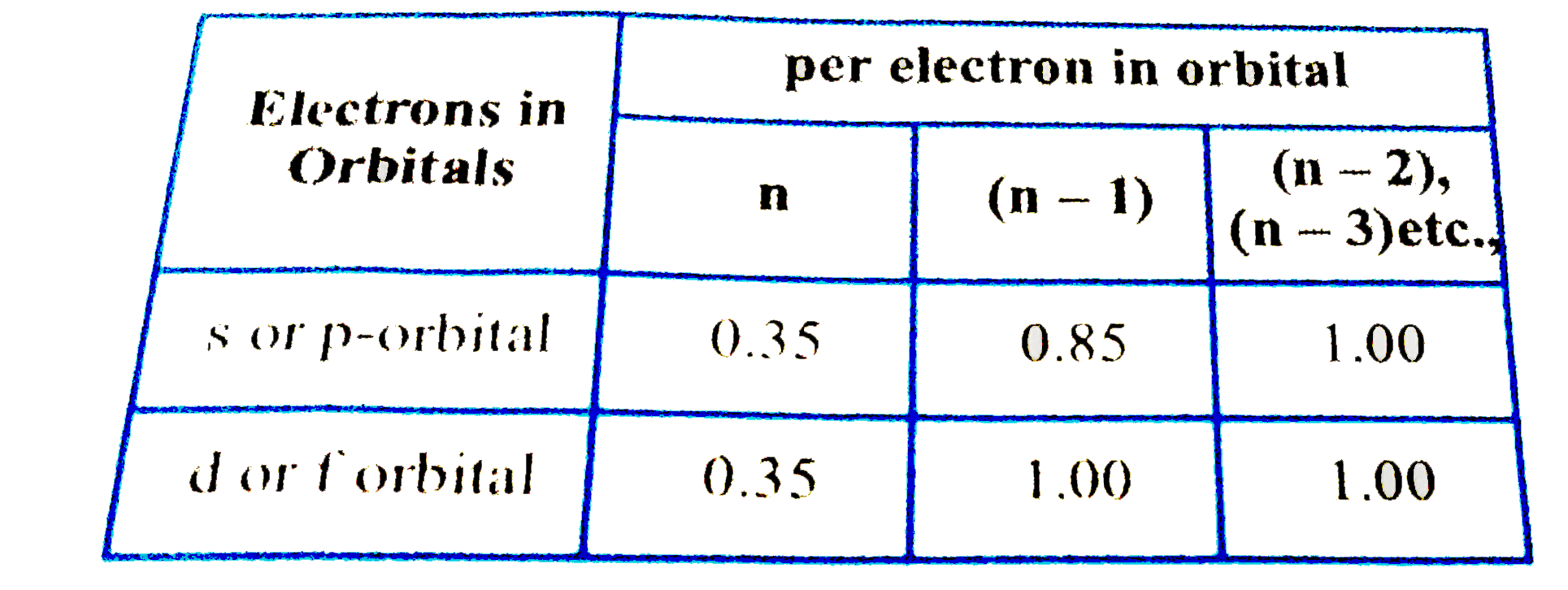
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type
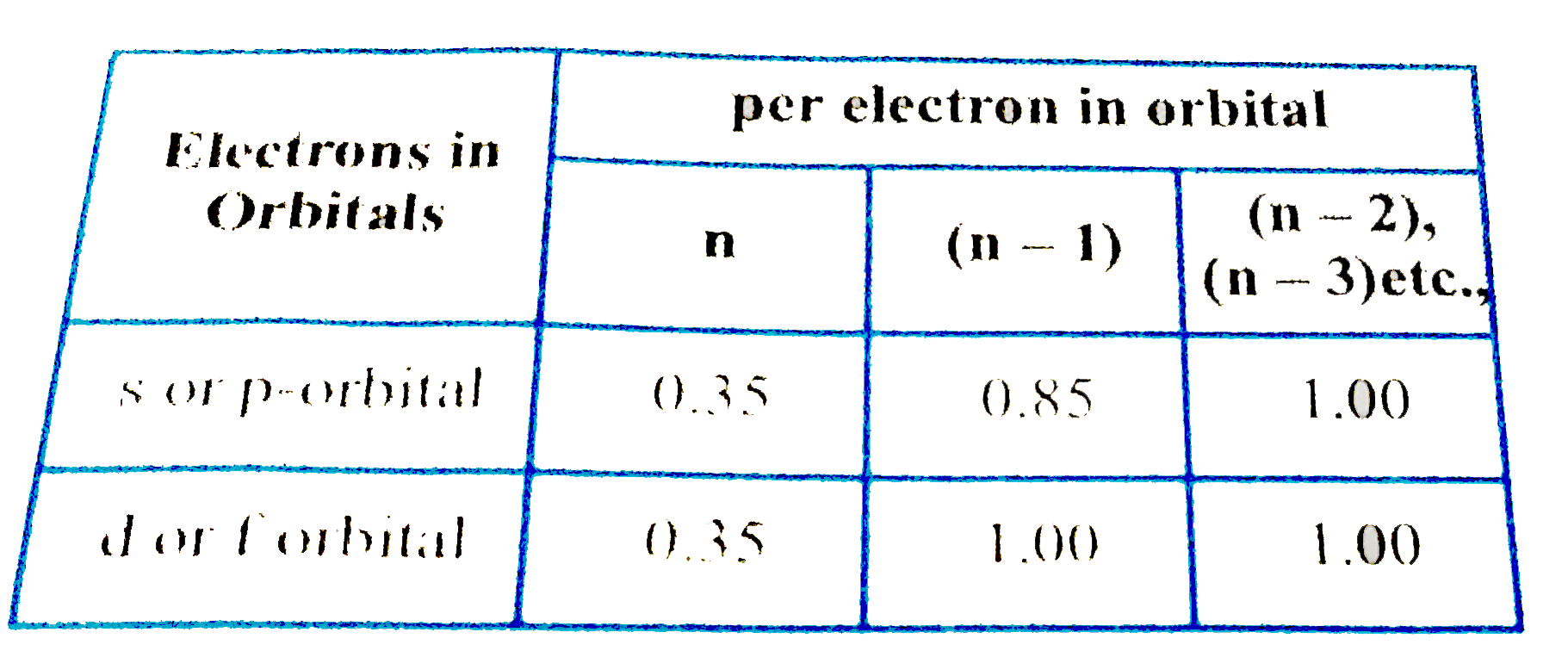
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type
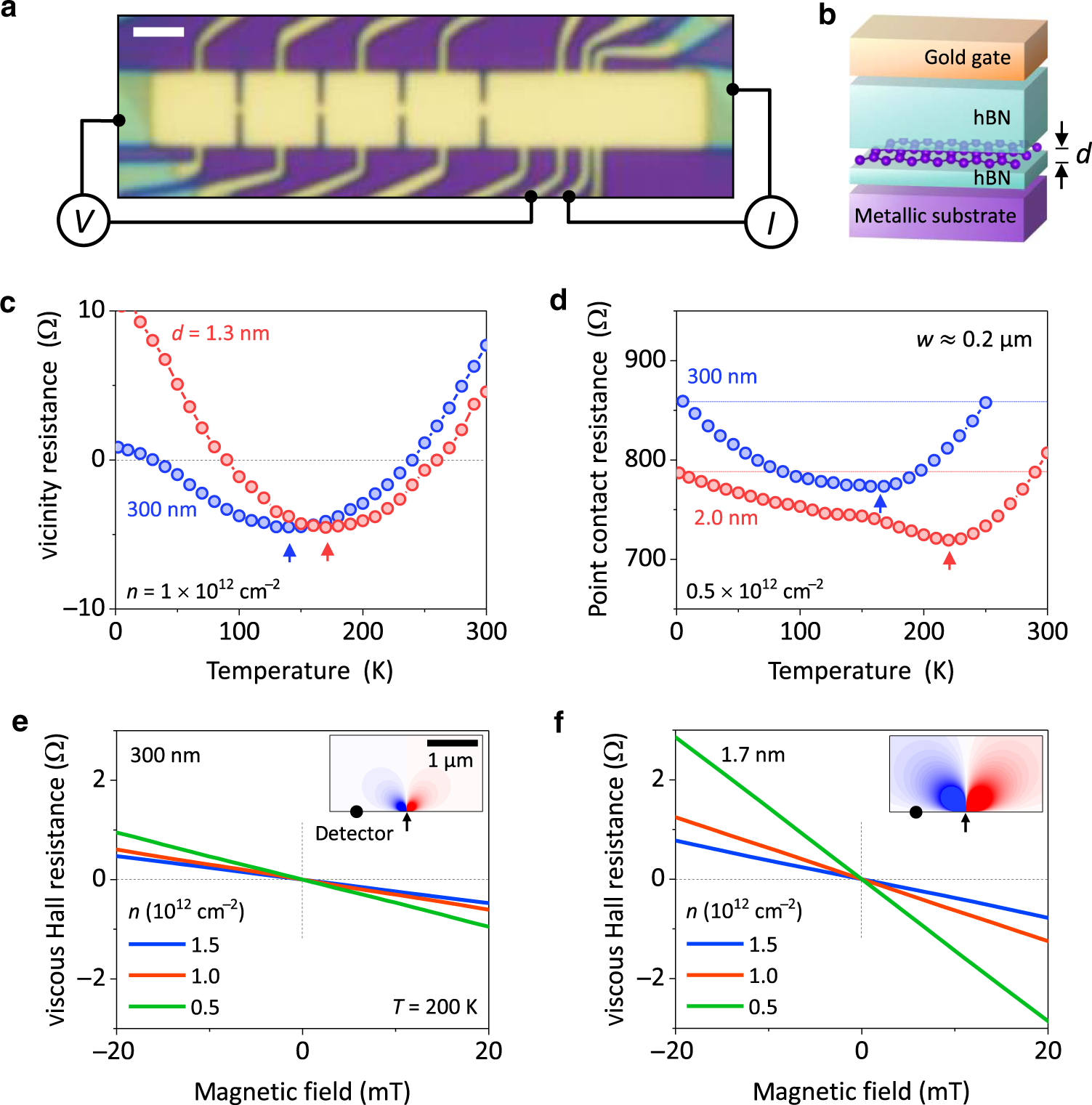
![PDF] Electron screening and excitonic condensation in double-layer graphene systems | Semantic Scholar PDF] Electron screening and excitonic condensation in double-layer graphene systems | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/12258383aed45faa8f3fb8f9277a44ffb3624dd7/1-Figure1-1.png)
PDF] Electron screening and excitonic condensation in double-layer graphene systems | Semantic Scholar

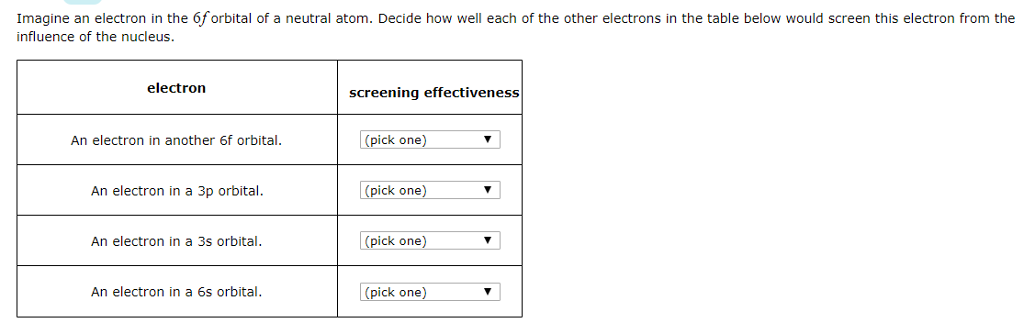
SOLVED: Imagine an electron in the 5d orbital of neutral atom Decide how well each of the other electrons in the table below would screen this electron from the influence of the

PDF) Electron screening effect in the reactions 3He(d, p) 4He and d( 3He, p) 4He Supported in part by INFN, BMBF (06BO812), DFG (436UNG113-146) and OTKA (T025465 | Matthias Junker -

Repulsive electron-electron interaction and nuclear charge screening: Ground state of two-electron atoms: Ndinya, Boniface, Akeyo, Joseph: 9783846540688: Amazon.com: Books